Any pursuit of science must be held up to the standards of testable hypothesis and experimental application. However plausible our propositions are, testability !
In this sense, Spectroscopy is the gold standard of experimental physics. In todays world, you would be amazed by the applications of Spectroscopy in your day to day. From identification of controlled substances to Tumor imaging, I daresay that spectroscopy has only left fewest of stones unturned.
What is Spectroscopy ?
The word Spectroscopy can be divided into two words; Spectra and Scope. Spectra refers to a phenomena that is continuous. Light is a spectrum with respect to its wavelength and frequency. Right from Gamma radiation through Visible region into Microwave region. Scope means to see, to visualize.
Although, light may be a spectra, the way it interacts, it is considered as particle. It transfers energy to the object in an all or nothing fashion. Absorption and emission of light by particles/atoms/molecules/ions leads to change in their energy levels which we call as transition between level or quantum states.
Let us entertain the idea of quantum states. Quantum states are discrete levels. For spectroscopy, it refers to levels of discrete energy. So, what about a two-level system ? A two-level system has two energy levels. They maybe and in most cases will be, marked by other identities, say Angular momentum or Spin etc. such that, a particle residing in that states will have those parameters as its own identities.
This two-level system lies at the heart of Spectroscopy. If we shine a light with energy equal to that of the difference in the energy levels, we would see a transition between the two states. A particle will go from lower energy level to higher energy level. Eventually, it will come back.
The part where it transitions and goes up is the section of Absorbance. The part where it comes down is the section of fluorescence. There is a lot more where that came from. X-ray spectroscopy, Microwave spectroscopy, UV-Vis spectroscopy etc. They deal exclusively in the energy ranges of transitions between states.
Can you see how easily we can summarize the phenomenon of spectroscopy ? Shine radiation and observe a transition. How do we observe a transition ? In the world of colourful molecules, the effect is apparent; Their colour. But that is not a quantifiable parameter for conducting experiments is it. We use a machine called as a Spectrometer.
The working of a spectrometer can be explained in a sequence chart. Basically, a light from a source, is allowed to pass through a holder containing the sample and a reference. A molecule/atom/ion in the sample should not be in the reference. Then, the light from both of them is collected and analysed using a grating. The sample would have absorbed segment of light from source and the light from sample would be devoid of that segment. Then, the lights are collected in a Photo-multiplier tube, which amplifies the incoming light signal. This information is then digitized, processed by the computer and the result is a plot of Intensity versus Frequency or Wavelength.
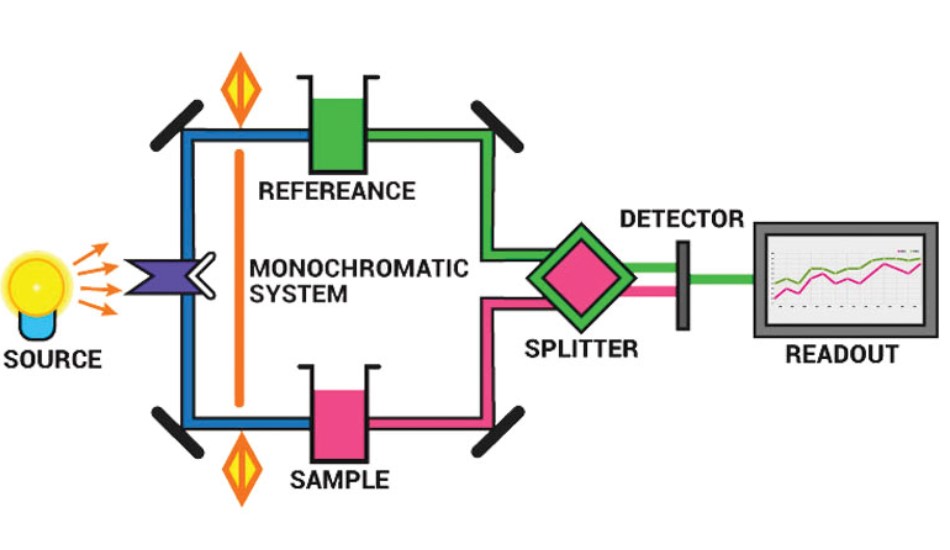
Applications of Spectroscopy
In chemistry, say that you have synthesized a compound. How do you understand its nature of bonding and the atoms it contains? You can narrow the field by limiting the reactants you have used for synthesis. We can use spectroscopy to identify all the particulars of that molecule through NMR and Infrared Spectroscopy. NMR (Nuclear Magnetic Resonance) spectroscopy, uses the energy states of Hydrogen nuclei and their transitions to identify the number of hydrogens in the molecule and the nature of atom it is bonded to. We also have Carbon NMR to identify the carbon content and the nature of carbon in the molecule; if it a double or triple bond or a aromatic system like benzene. The IR spectroscopy is special.
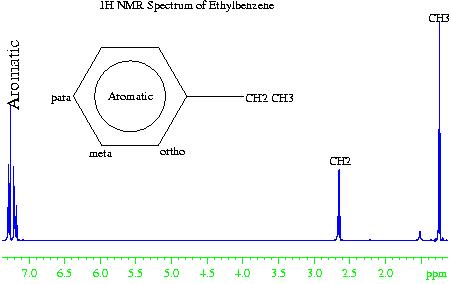
The energy of IR radiation matches the energy of vibration of atomic bonds. The wiggles of bonds of any atom can be identified using its characteristic IR region.
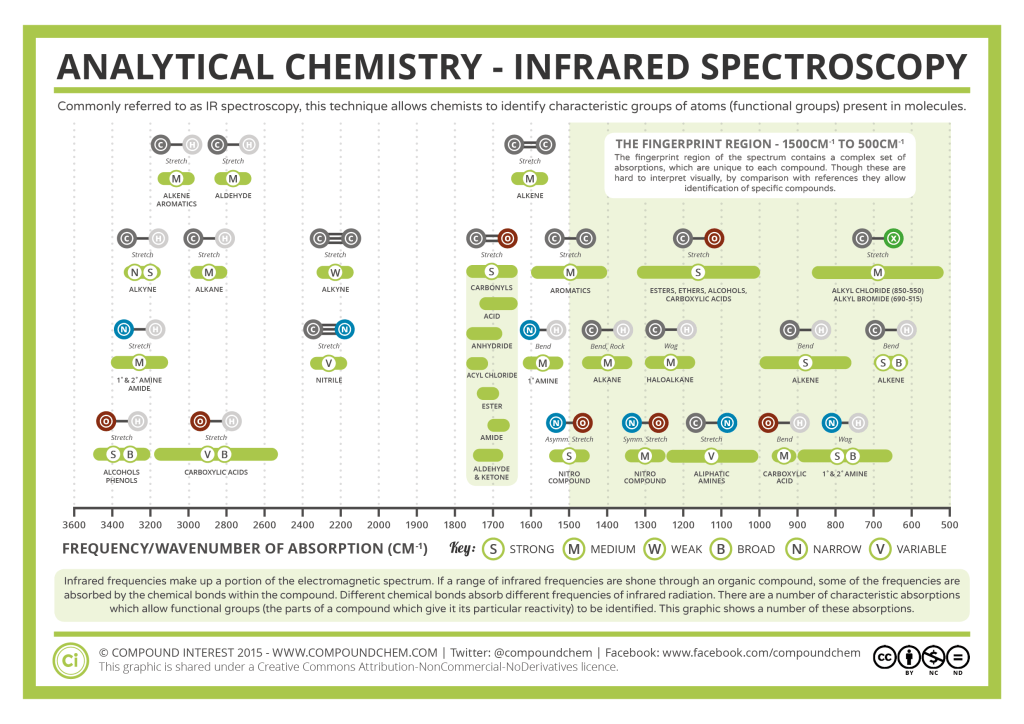
Can you extrapolate the usage of Spectroscopy in bomb detection and drug detection now ?
A pro-active bomb squad will try to invent and characterize potential bomb markers. All their identities will be actively used in security search in Airports and other hot zones. Same thing is done in Drug enforcement.
We can even use spectroscopy to identify relative concentration of the molecule in a solution. The height or the intensity of the peak is directly proportional to concentration. That is how purity is determined by spectroscopy.
This is kind of illustrated in Breaking Bad, an AMC sitcom. When Mr.White produces the first batch of his Methamphetamine, Mr.Jesse remarks that the nature of product is glass grade and that Mr.White is an artist. What he means to say is that, the product obtained is so clear optically, that it should be very pure. Mr.Jesse has not performed any quantitative analysis but his eye is a very crude spectrometer.
All the MRI, PET, CT and X-rays are merely examples of spectroscopy. If you have time and a reliable spectrometer, you can have a lot of fun by synthesizing new substances and characterizing.
This is my wit’s end introduction to spectroscopy. We can expect a more specific articles from my esteemed colleagues in future.